|
|
|
Also available : CHIME Version
What is frontalin ?
Frontalin, first isolated by G. Kinzer [1], play an important role as volatile signal in sytems of chemical communication among many insect species. This is an aggregation pheromone of a beetle population in the scolitidae family :Dendroctonus adjunctus Blandford ; Dendroctonus brevicomis ; Dendroctonus frontalis Zimmermann ; Dendroctonus ponderosae Hopkins ;
Dendroctonus pseudotsugae Hopkins ; Dendroctonus terebrans (Olivier).
Among these insects, the Dendroctonus frontalis Zimmermann beetle called Southern Pine Beetle (SPB) is the most destructive
to pines forest in the southeastern United States.
|
|
|
The molecule of frontalin
Frontalin contains two asymmetric centers. K. Mori [7], [8] has shown that the absolute configuration of the biologically active species is (1S, 5R).
|
|
(-)-frontalin, the structure of which shown left, is a tricyclic molecule with an acetal linkage. |
|
|
Biological aspect : mechanism of tree colonization
Initial attack
The aggregation mechanism of these insects isn't perfectly known but it seems that the volatil compounds producted by the trees attract some female insects (the main constituant
of the volatil compounds is a monoterpene called a-pinene). The females create a mixture of (-)-frontalin, trans-verbenol and verbenone. These products have to effect to attract a lot of other male and female insects.
The trans-verbenol and the verbenone intensify the attracted character of (-)-frontalin.
Mass attack
After the initial attack and during three or five days, males and females reproduce and continue to produce (-)-frontalin, trans-verbenol and verbenone. When a concentration threshold of the two last
substances is obtained, these ones inhibit the (-)-frontalin and then stop the aggregation.
 |
 |
 |
 |
|
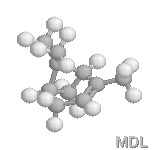
a-Pinene |
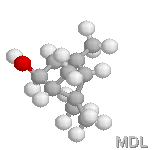
trans-verbenol |
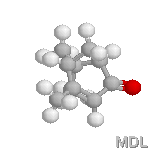
Verbenone |
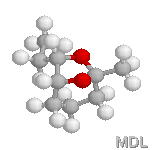
endo-Brevicomine |
You can save the molecule file with the right mouse button. You will be able to see the molecule without connection with a visualization program like RasMol.
The complete biosynthetic pathway for frontalin is not yet known. A possible way is the transformation of isoprenoid compounds which is regulated by the enzyme HMG-CoA reductase.
On account of the difficulty to isolate these natural products and of the importance of chemical mediators, several chemists have realized the synthesis since about thirsty years. The first strategies have rested on the
racemic synthesis and more recently they rest on the enantioselective synthesis of (-)-frontalin.
Retrosynthetic analysis
The retrosynthetic analysis is shown below :
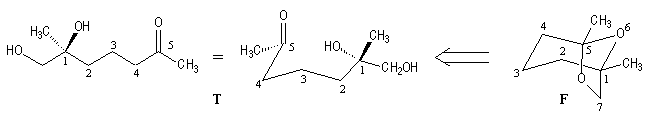
Since (-)-frontalin (F) can be viewed as being formed by internal acetalization of the dihydroxyketone (T). This precursor contains only one asymmetric center (1S). Although frontalin contains two asymmetric carbon.
The configuration at C5 is dictated by the (1S) center during the formation of the acetal linkage.
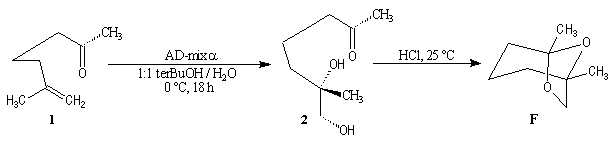
Synthesis of racemic frontalin
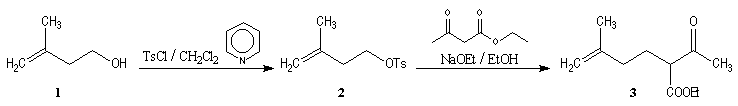
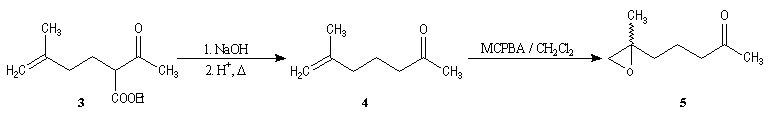
The key reaction is the opening of epoxyd catalysed by an acid with the participation of a neighbouring carbonyl group.
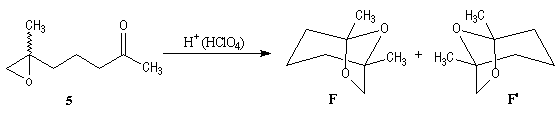
Enantioselective synthesis of the (-)-frontalin
A number of enantioselective syntheses of both (+) and (-)-frontalin have been reported. Three exemples will be given here.
M. C. Trinth, J-C Florent, C. Monneret [4] ; [5] used a derivative of lactose as chiral substrate.
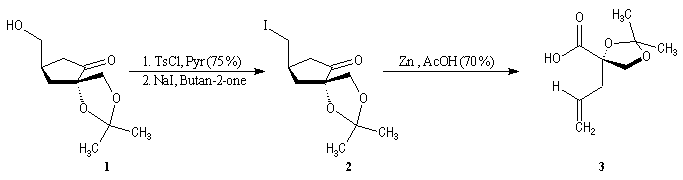
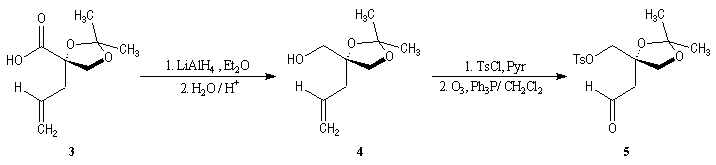
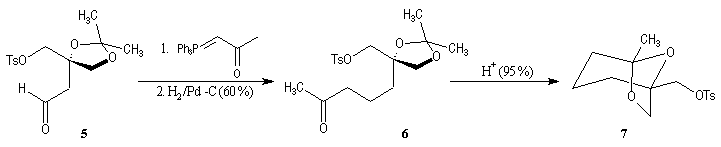
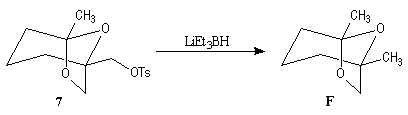
First step is the preparation of the a-siloxyketone (2).
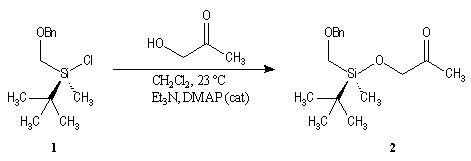
p facial selectivity under the influence of the chiral (tert-butyl)(methyl)(benzyloxymethyl)silyl group is not very high. The addition of Grignard reagent G to a-siloxyketone (2) leads with only low stereoselectivity to the alcohols (3/3') (60 : 40) precursors of frontalin F.
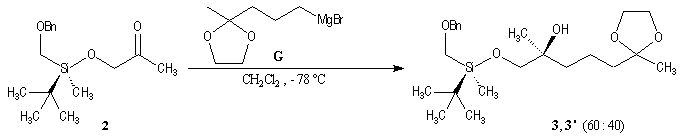
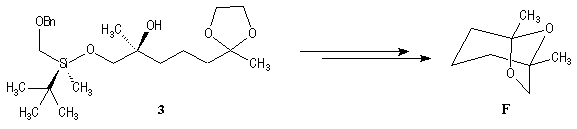
This methodology gives frontalin with 60-70 % ee.
Bibliography
[1] G. W. Kinzer; A. F. Fentiman T. F. Page; R. L. Foltz; J. P. Vite; G. B. Pitman Nature 1969, 221, 447.Links
Synthesis
[9] Synthesis of frontalin using a Chiral Silyl Ether
[10] TP Université de Liège
[11] Frontalin Project Home Page
Biosynthesis
Biosynthesis of frontalin in the Jeffrey Pine Beetle
Biosynthesis of bark beetle aggregation pheromones
Enzymatic and microbial hydrolyse of epoxydes
Asymmetric hydrolysis of epoxydes
Biological aspects
Attractive and inhibitory pheromones produced in the bark beetle Dendroctonus Brevicomis
Chemical ecology of the Southern Pine Beetle
SPB Update. Utilisation de la verbenone
The Southern Pine Beetle
For educational purpose - No commercial use
Page created by Gérard Dupuis - Lycée Faidherbe LILLE
gdupuis-prof@faidherbe.org
This site was last updated December 2000