Triphenylmethyl radical : properties and synthesis
A hundred years ago, the discovery of the first organic radical
by Gérard Dupuis and Nicole Berland
Lycée Faidherbe - LILLE
Also available : HTML Version
Summary
A hundred years ago, when he tried to make the synthesis of the hexaphenylethane, M. Gomberg found out the first organic
radical : the triphenylmethyl radical. From this period, this radical has been studied by several rechearch teams. For example,
we have seen its particular propeller shape and we can have interpreted its stability by steric factors. At the ordinary temperature, an equilibrium exists
between the radical and a dimer which isn't the hexaphenylethan. An experiment, easy to realize, can show this equilibrium and is proposed in the
following text.
The discovery of the triphenylmethyl radical
The year 2000 was the 100th anniversary of the publication by the American chemist (originate from Russia) Moses Gomberg of the
experimental discovery of an organic radical : the triphenylmethyl radical.
M. Gomberg studied the alkanes substituted by phenyl groups. In 1897, when he was working in the Victor Meyer laboratory in Heidelberg, he succeeded
the first synthesis of tetraphenylmethane. Then, in the university of Michigan, using the Wurtz's reaction, he tried
to prepare the hexaphenylethan by the reaction of triphenylmethyl chloride with silver metal in the benzene as solvent. The reaction he tried to realize was :
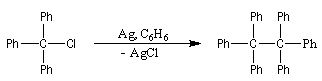
When he was realizing the reaction in a round bottomed flask with an inert gas : CO2, M. Gomberg obtained a yellow solution with odd properties :
- On heating, the yellow colour increased and, on cooling it faded.
- After the evaporation of the solvent, a white solid A appeared, and in disolving in benzene under CO2 atmosphere, the yellow coloured solution with the
same properties appeared again.
- If the air took place of CO2 in the flask, the yellow colour rapidly faded and after
the evaporation of the solvent, a white solid B appeared. In dissolving B in benzene, a colourless solution appeared.
The massic percentages of the elements in A and B were different.
|
Conditions |
Compounds |
MP (°C) |
Composition |
|
with air |
B |
185 |
C : 88 %, H : 6 %, O : 6 % |
|
with CO2 |
A |
147 |
C : 93,8 %, H : 6,2 % |
Gomberg concluded that the yellow solution was constituted by hexaphenylethan A and
that B was the triphenylmethyl peroxyde. To explain the formation of A and
B depending on the experimental conditions, Gomberg supposed that A was in
equilibrium with an unknown composed in this period : the triphenylmethyl radical (reaction 1).
With air, the radical reacted with O2 that explained the formation of B (reaction 2).
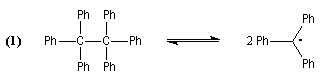
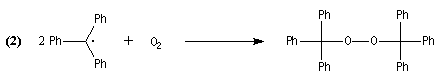
In 1900, Gomberg wrote an item in wich he described this discovery and proposed the previous interpretation. This item was published in the American Chemical
Society Journal and in the Berichte der Deutschen Chemischen Gesellschaft where it was named [1] :
"Triphenylmethyl ein Fall von dreiwertighem Kohlenstoff"
That significated : "triphenylmethyl, a case of trivalence for the carbon". The idea that the carbon element could be trivalent, wasn't well accepted by the
scientifical community in this period.
Who is Moses Gomberg ?
Moses Gomberg was born on February 8th 1866 in Elisabetgrad in Russia. In 1884, his family moved to Chicago. Gomberg studied in the University of Michigan and passed a doctorate in
1894. He lived from 96 to 97 in Germany where he worked first in A. Baeyer laboratory in Munich and then in Victor Meyer laboratory in Heidelberg. After his return to the
University of Michigan, he studied the alkanes substitued by phenyl groups. Moses Gomberg died on February 12th 1947.
The structure of the triphenylmethyl radical
A radical is a molecule (or an ion) which has one or several non shared electron. Nowadays, a lot of proofs shows the reality of this radical. Ultraviolet analysis shows that this radical isn't planar;
this result is confirmed by the electronic-diffraction : the angle between phenyl groups is about 35°.
This particular shape can be explained by a compromise between a planar conformation with a maximum
delocalization of the electrons and a repulsion of the hydrogen atoms localised in the ortho positions
of the phenyl groups.
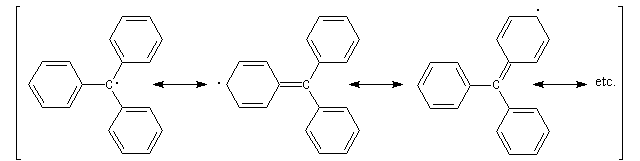
Mesomeric forms of the triphenylmethyl radical
Lifetime for the triphenylmethyl radical
Radicals are often intermediates of reactions and their lifetime is generally very short [4].
They can be destroyed in different ways :
- by dimerisation
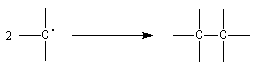
- by dismutation
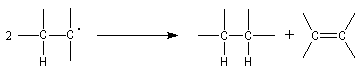
- they react rapidly with oxygen and form peroxydes
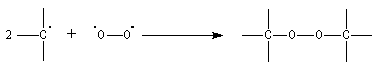
The interesting equilibrium is :
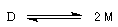
M represents the triphenylmethyl radical and D the dimer. The experiment shows that the equilibrium constant of the
reaction is K0 = 2 10-4 at 25 °C in the benzene as solvant [4], [5]. This constant
is low but sufficient to detect the radical. An interesting problem is to know what is the origin of the relative stability of this radical.
We can interpret it by the stabilization of the final state due to the electronic delocalization in the radical or by the destabilization of
the initial state due to an important steric hindrance in the dimer. This previous equilibrium has been studied with different radicals like (I)
or (II) represented below.
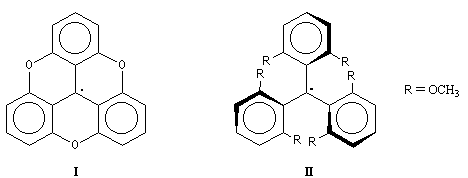
- In the first one (I), the oxygen bounds impose a planar structure permitting a maximum delocalization of the
electrons.
- In the second one (II), on the contrary, the repulsion of methoxy groups in ortho imposes a non planar structure and this fact
reduces the delocalization.
If the delocalization factor is the most important, the dimerization of (I) must be difficult and we must observe little dimers in the equilibrium. But
if the steric factor is the most important, it's (II) that gives little dimers. The experiment shows that for (I), the dimer
is in a huge majority and that for (II), the radical is in a huge majority. So the major factor is the
steric factor [6].
Dimer is not hexaphenylethan
Gomberg taught that the dimer was the hexaphenylethan. But in 1968 the real structure of this dimer was known. The RMN spectrum of the dimer solution has these characteristics [7] :
|
Protons |
Aliphatic |
Olefinic |
Aromatic |
|
d (ppm) |
5 ppm (1p) |
5,8-6,4 (4p) |
6,8-7,4 (25p) |
This spectrum is explained by a dimer "head-tail".
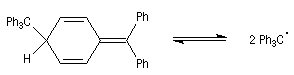
How can we show the equilibrium ?
Principle of the experiment
The radical is shown by its yellow colour in solution. At 20 °C, the equilibrium constant
is K0 = 10-4, the solution having mainly the dimer isn't coloured.
K0 depends on the temperature with the following relation of Van't Hoff :
dln K0/dT = DrH0/RT2
The reaction is endothermic : DrH0 > 0 and then K0 increases when the temperature rises.
And so if we increase the temperature of the previous solution the concentration of the radical rises and the yellow colour too. But if we decrease the temperature
the opposite observation appears. To show this equilibrium we have to operate without air to provide the formation of the peroxyde.
The experiment
|
|
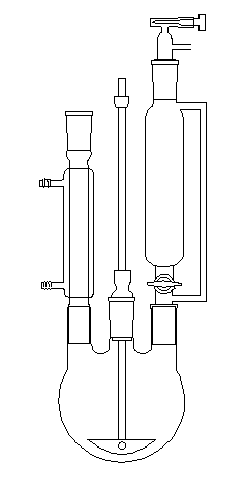
The experimental assembly is the same as this used for the synthesis of a Grignard reagent. The vessel has to be clean and dry. We use a three-necked round bottomed flask with
a condenser, a cylindrical funnel with pressure-equalising permitting the introduction of an inert gas, and a stirrer (the stirrer can be omitted and the agitation effected with a magnetic stirrer).
In the round bottomed flask introduce m = 0,1 g of Zn dust. Introduce in the cylindrical funnel, a solution with 0,2 g of triphenylmethyl chloride in 100 mL of cyclohexan. Introduce with the cylindrical
funnel an inert gas like CO2.
Remark : the radicals are electricaly neutral and it's logical to use an apolar aprotic solvent. Gomberg used the benzene but nowodays, due to the danger of this product, we prefer the cyclohexan.
Introduce gently the solution contained in the funnel into the round bottomed flask. After some minutes a yellow colour appear. Stir the solution during about 15 min.
|
The experimental assembly for the synthesis of the triphenylmethyl radical
Remove the cylindrical funnel and with a pipette take out a small volume of the solution (without taking zinc which must stay in the bottom of the
flask). The solution obtained is quite colourless due to the low temperature.
Put the solution in a small conical flask, close this one with a cap and a long glass tube used for air condenser.
- Immerse the flask in hot water. The yellow colour increases.
- Immerse the flask in cold water. The yellow colour fades.
Necessary products
- Zn dust
- Triphenylmethylchloride Ph3CCl (TF : 110 °C, 112 °C). Be careful : dangerous product.
- Cyclohexan (TF : 6,5 °C, TE : 80,3 °C)
Conclusion
The synthesis of the triphenylmethyl radical permits to realize an experiment with the same
assembly that this one used in the synthesis of a Grignard reagent. It insists on the necessity of an inert gas.
It permits easily to show an organic radical. This experiment shows, at last, the influence of the temperature on an equilibrium.
References
[1] Gomberg. J. Am. Soc. 22, 757 (1900).
[2] G. N. Lewis, D. Lipkin and T. T Magel, J. Am. Soc. 66, 1579 (1944).
[3] P. Anderson, Acta Chem. Scand. 19, 629 (1965).
[4] F.A Carey, R.J Sundberg Advanced Organic Chemistry (Plenum Press 1990).
[5] M. Julia, Intermédiaires de réaction en chimie organique : les radicaux (BUP 570 décembre 1974).
[6] Sabacky et coll. J. Am. Soc. 89, 2054 (1967).
[7] J. March, Advanced Organic Chemistry (John Wiley, 3 ed).
[8] H. Lankamp, W. Th. Nauta et C. MacLean, Tetrahedron Lett. 249 (1968).
[9] G. Dupuis, Bulletin de l'Union des Physiciens Bulletin de l'Union des Physiciens N° 840 janvier 2002.
[10] Intermediates to Stable Compounds, Science 2002 295 : 1846-1847.
Links
The tripenylmethyl Radical by A. Hudson and R. Jackson - University of Sussex
Gomberg Lecture
[HOME PAGE]
For educational purpose - No commercial use.
Page created by Gérard Dupuis - Lycée Faidherbe LILLE.
gdupuis-prof@faidherbe.org
This site was last updated February 2005.
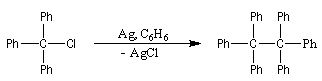
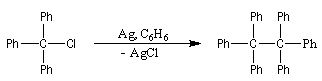
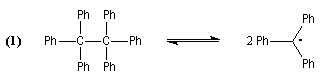
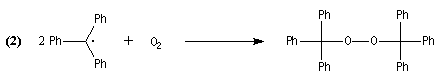

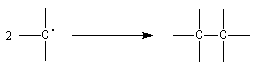
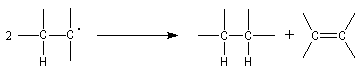
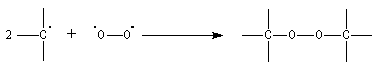
![]()

